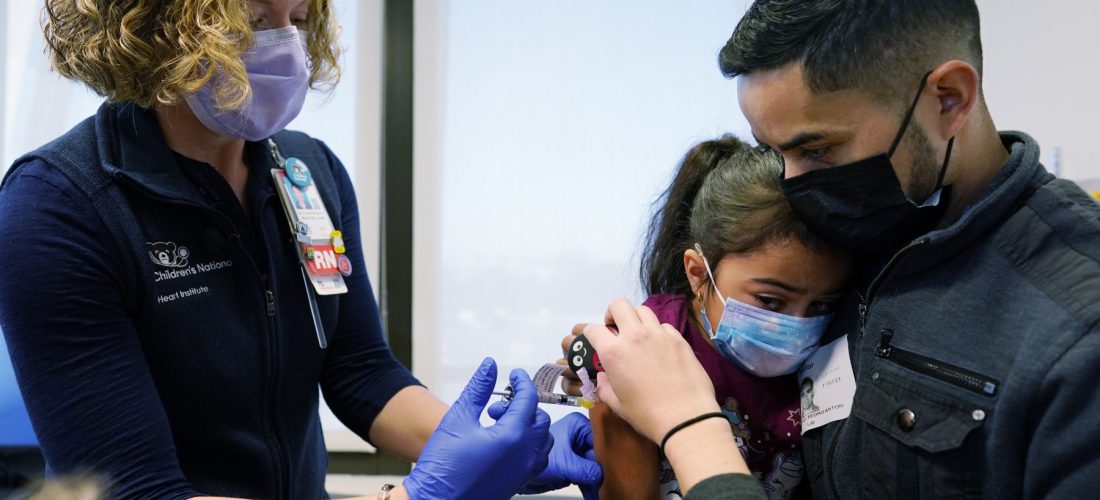
(Silver Spring, MD) — The FDA is postponing a meeting that was supposed to go over data from trials of COVID vaccines in young kids. The agency said it needed more time to consider new data and to allow for transparent “public discussion” as they mull over approval for the vaccines. This comes after Pfizer and BioNTech delayed their request for the FDA to authorize the vaccine for children under five-years-old. The trial for children between six months and four-years-old is ongoing, with data being shared with the FDA as it progresses.